Surface coat proteins on Plasmodium, the parasite that causes malaria, provide new targets for a malaria vaccine.
3 Bullets:
- ISB scientists collaborate with scientists from Center for Infectious Disease Research and Johns Hopkins University to identify new proteins on the surface of the malaria parasite Plasmodium.
- The research aims to provide new targets for a malaria vaccine.
- It is discovered that some Plasmodium surface proteins have sugar attachments that can cloak these proteins from the human immune system.
By Dr. Kristian Swearingen
A new report in PLOS Pathogens describes the results of a collaboration between researchers at ISB, Seattle’s Center for Infectious Disease Research (CIDResearch), and Johns Hopkins University. We used mass spectrometry-based proteomics to identify dozens of proteins that were previously not known to be present on the surface of Plasmodium falciparum, one of the Plasmodium species that causes the disease malaria in humans. Because surface coat proteins can potentially be recognized by the body’s immune system, the proteins we have identified are promising targets for new vaccines against malaria. Additionally, we discovered that two of these surface coat proteins are modified with attached sugars that change the way the proteins are recognized by the immune system and may affect the way that future vaccines are designed.
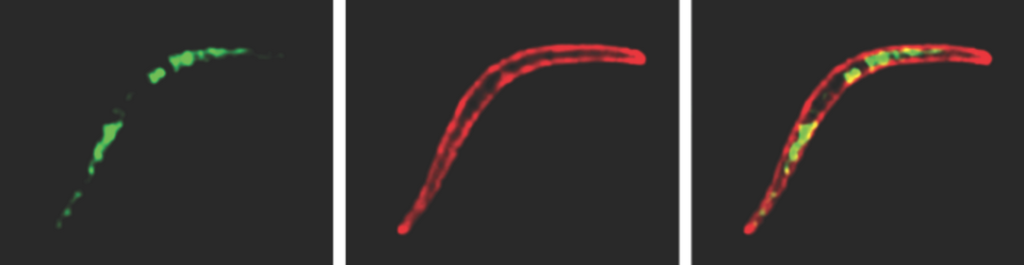
Screen shot from Figure 1B. Transgenic salivary gland sporozoites expressing p38 with an HA tag were subjected to an indirect fluorescence assay (IFA).
The disease malaria is caused by parasites of the genus Plasmodium. According to the WHO World Malaria Report, there were as many as 300 million new infections and half a million deaths in 2015. More than half of these deaths were children under five years of age. Among the reasons malaria remains such a formidable disease is the lack of an effective, approved vaccine. The most advanced malaria vaccine candidate, called RTS,S, showed approximately 50 percent efficacy in phase-III clinical trials. While these results are very encouraging, a better vaccine is needed if malaria eradication is to be achieved. The RTS,S vaccine contains a portion of a protein found on the surface of P. falciparum sporozoites, the form of the parasite that is transmitted from mosquitoes to humans. Exposing the immune system to this protein trains the body to recognize and fight sporozoites when they arrive. It is thought that the power of this vaccine could be boosted if it were combined with several other sporozoite surface proteins to create a multivalent vaccine. However, only a handful of these surface proteins are currently known.
In order to identify new targets for a multivalent vaccine, we undertook the most comprehensive effort to-date to identify proteins found on the surface of P. falciparum sporozoites. The parasites were raised in the laboratories of Professors Stefan Kappe at CIDResearch and Photini Sinnis at Johns Hopkins University. Over the course of the experiment, literally thousands of Plasmodium-infected mosquitoes were dissected one-by-one in order to extract the salivary glands and isolate the Plasmodium parasites. The parasites were then exposed to a compound that attached a chemical tag only to proteins on the parasite surface. This tag enabled us to isolate surface proteins from the rest of the parasite material so that we could identify them using the state-of-the-art mass spectrometry equipment in the lab of Professor Robert Moritz at ISB.
Our work identified dozens of new sporozoite surface proteins. Several known proteins were also identified, such as the component of RTS,S, as well as many new proteins which had not previously been known to be on the sporozoite surface. Excitingly, we also discovered that some of these surface proteins are modified in a way that no one had known before. After a protein is produced in a cell, it can be modified with other biomolecules in order to alter its function. One such modification is glycosylation, the attachment of sugar molecules directly to the protein. Because mass spectrometry enables us to detect even the smallest changes in a protein’s composition, we were able to demonstrate for the first time that two of the most abundant proteins on the sporozoite surface are modified with sugars. One of these proteins is the component of the RTS,S vaccine. This finding is important because attaching sugars to proteins changes their shape and affects how they are recognized by the immune system. If the immune system is trained to recognize an unmodified protein, it may not recognize the glycosylated protein when it encounters a parasite. Future vaccine designs will need to account for protein glycosylation.
Funded by the Bill & Melinda Gates Foundation, these new insights into the biology of a deadly parasite provide valuable new information to vaccine researchers working to eradicate malaria.
Title: Interrogating the Plasmodium Sporozoite Surface: Identification of Surface-Exposed Proteins and Demonstration of Glycosylation on CSP and TRAP by Mass Spectrometry-Based Proteomics
Journal: PLOS Pathogens
Authors: Kristian E. Swearingen, Scott E. Lindner, Lirong Shi, Melanie J. Shears, Anke Harupa, Christine S. Hopp, Ashley M. Vaughan, Timothy A. Springer, Robert L. Moritz, Stefan H. I. Kappe, Photini Sinnis
Link: http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1005606


 moritz.isbscience.org/research/scratching-surface-malaria-parasite/
moritz.isbscience.org/research/scratching-surface-malaria-parasite/